Pivot-TR bridge received approval for EFS to treat TR
December 2022
Tau Medical Inc.
Tau Medical announced receiving the approval of the Korean Ministry of Food and Drug Safety (MFDS) for clinical study of its TTVI (Transcatheter Tricuspid Valve
Intervention) implantable spacer, Pivot-TR to treat TR (Tricuspid valve Regurgitation).
The study is using the Pivot-TR to treat patients with torrential and severe TR (Tricuspid valve Regurgitation) by blocking the regurgitation and to check the safety
and stability of the device after implantation for one week. After one week of implantation, the device will be retrieved by pulling it out with catcher catheter
or surgery.
The study will be done with 7 patients in eight medical centers in Korea.
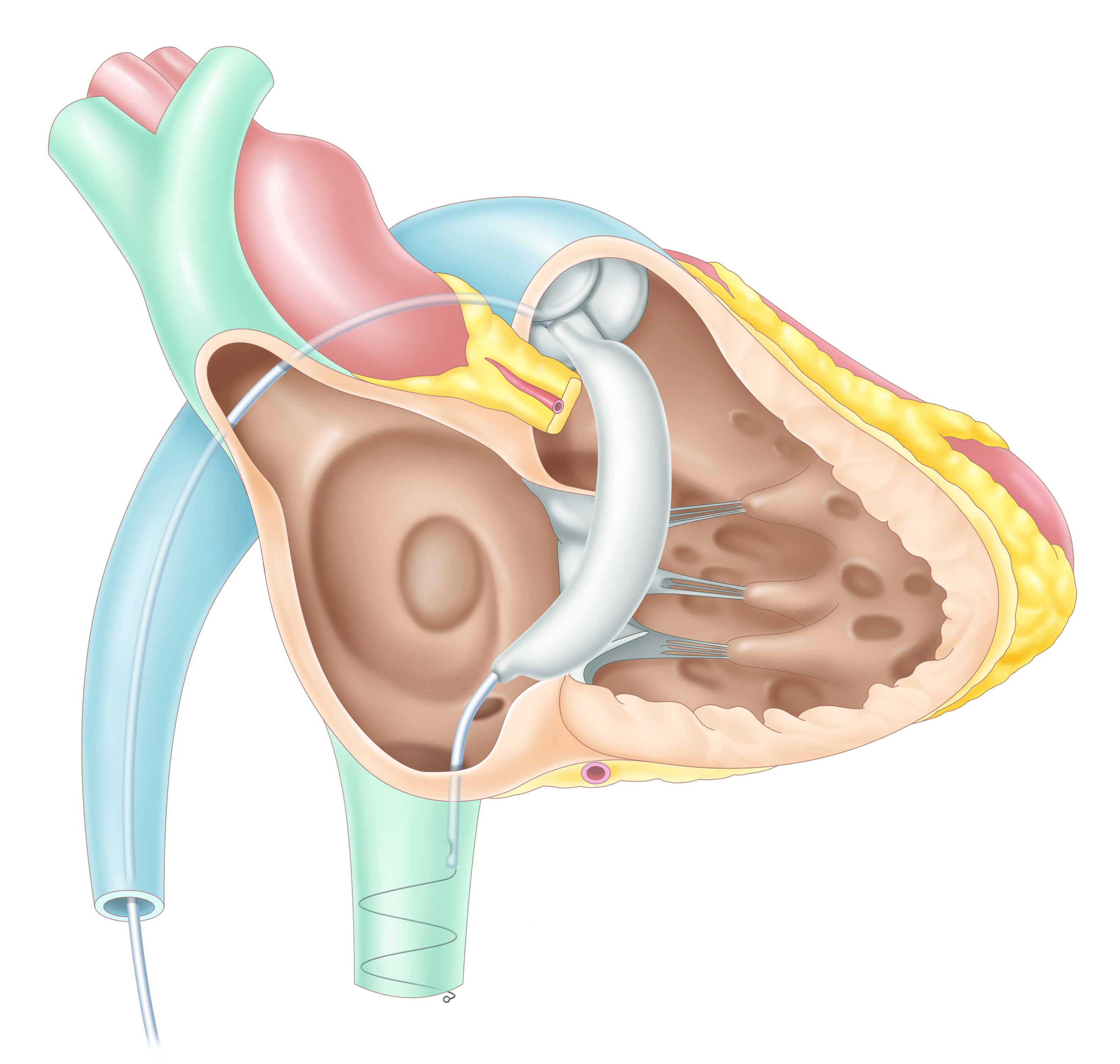
[Pivot-TR]
The Pivot-TR is a three-dimensional spacer that blocks the blood regurgitation by passing through the valve coaxially, which can be delivered
by minimally invasive catheters in a simple procedure. Due to simple and atraumatic anchors such as a long nose and a spiral tail, it can be delivered easily
within several minutes and retrieved if necessary.
The Pivot-TR can be categorized into three versions: the Pivot-TR temporary is for checking the heart’s durability to the mitigated TR before open surgical repair.
The Pivot-TR bridge can be implanted for less than four weeks to mitigate the severe TR to mild TR, allowing patients to be safe enough to get open surgical repair.
The Pivot-TR permanent is a permanently implantable version to treat the TR without open surgical repair. The Pivot-TR temporary is under EFS in KR,
the Pivot-TR bridge is just about to start the EFS in KR, and the Pivot-TR permanent is preparing for IDE (Investigational Device Exemption) submission to the U.S. FDA
[Tau Medical Inc.]
Tau Medical is developing trans-catheter devices for structural heart diseases like MR (Mitral valve regurgitation) and TR (Tricuspid valve regurgitation).
Tau’s Cerclage MR is well known as an innovative annuloplasty treatment for MR and enlarged LV reshaping, and is now under clinical study.
link
| 이전글 | EFS of Pivot-TR temporary was finalized | 2023-01-09 |
| 다음글 | RF ablation received approval for EFS to treat a.fib | 2022-10-25 |
